Understanding humidity and dehumidifiers
This is a short introduction to understanding the basics of humidity and dew point when selecting Rosahl dehumidifiers.
To minimise condensation when dehumidifying with Rosahl or other devices it is best to reduce the relative humidity in the enclosure before reducing the temperature otherwise the dew point will be too high and condensation will occur .
Humidity
Water vapour is a gas and mixes freely with the nitrogen and oxygen in the air. Like most gases it also has a liquid form-water.
Humidiity refers to the amount of water vapour in air. Factors that can affect the humidity include the available moisture, temperature and atmospheric pressure. There are several different forms of humidity: absolute; specific, and relative humidity. We are considering only relative humidity (RH)
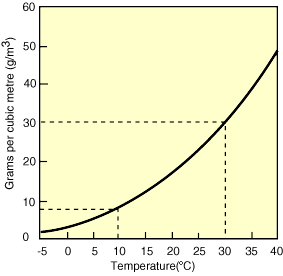
The saturation point of air is maximum level of water that it can carry (100%), and this is dependant on the temperature of the air. Water densities in air are usually given in g/m³, and saturated air at 10 ºC will hold 9.4g of water per m³, whereas at 40ºC it will hold 51.1g (see graph below).
Relative humidity (RH)
Relative humidity (RH) is the relationship between actual water density and saturated water density as follows:
RH=actual water vapour density/saturation water vapour density x 100%
From this equation it is possible to calculate any value given the other two. For example, if the temperature is 20ºC and the RH is 57.8%, then the actual water vapour density is 57.8 x 17.3/100=10g/m³
Dew point
If the air temperature continues to fall the relative humidity will rise until it reaches 100%. This is its saturation point and if the temperature falls further it releases the moisture as condensation.
Finally, for more information on relative humidity go to HyperPhysics